PFS
Introduction
A second allogeneic transplantation (allo- SCT) in patients who relapse after first-line autologous transplantation (auto- SCT) is mentioned in a recent statement of the European Society for Blood and Bone Marrow Transplantation (EBMT) as a valuable clinical option, especially when novel immunotherapies such as bispecific antibodies or CAR -T cells are not available. However, it is still unclear whether allo- SCT achieves better results compared to auto-SCT in patients who relapse after first-line treatment with auto-SCT.
Objectives
To compare progression-free (PFS) and overall survival (OS) between allo-ASCT and auto-ASCT as second-line therapy in patients who relapsed after first-line therapy with auto-SCT by analyzing individual patient data and extracting results from published reports when individual patient data were not available.
Methods
We searched the electronic databases PubMed, EMBASE, and the Cochrane Library and Up to Date. The search was limited to English-language publications from 1995 to February 2022, and only 6 studies were found that met the requirements for comparing allo SCT and auto- SCT as second-line therapy for patients who had relapsed after the first Auto- SCT. Individual patient data on survival were available for 512 patients from the Japanese Society for Hematopoietic Cell Transplantation registry data (Ikeda T et al. Hematol Oncol 2019) and for PFS and OS for 289 patients from the CIMBMTR registry (Freytes CO et al., BMT 2014). For the other 3 studies, which included 170 patients, data were extracted from the original publications (Mehta J et al, BMT 1998, Qazilbash M et al, Cancer 2006, and Wirk B et al. J Clin Res 2013). We used standard meta-analysis techniques to calculate pooled estimates from random-effects models as incorporated in the software Review Manager V. 5 (The Cochrane Collaboration). Fixed-effect models used as sensitivity analyzes yielded similar results (data not shown). I² = [(Q - df)/Q] X 100%, where Q is the chi² statistic for heterogeneity and df is the degrees of freedom, in order to quantify inconsistency between study outcomes.
Results
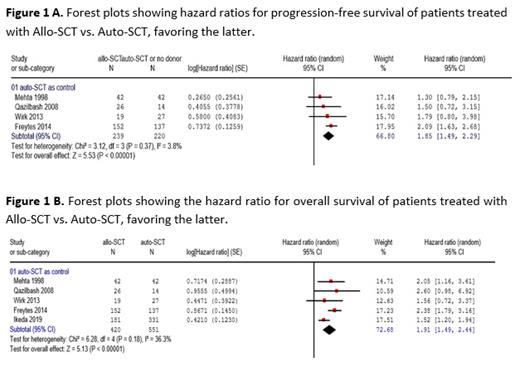
All comparisons of included studies were retrospective in nature, with one study using a cross-case approach (Mehta J et al). Conditioning regimens used for allo-SCT varied between reduced-intensity conditioning (Freytes CO et al and Qazilbash MH et al) and ablative regimens (Mehta J et al). Our analysis was based on individual patient data from 801 patients and data from 170 patients from published reports. No data on PFS were available in the study by Ikeda T et al.
The results showed a HR of 1.85 (CI, 1.49-2.22, p < 0.00001) for PFS in favor of the auto-SCT group (Figure 1A). No relevant heterogeneity was observed between reports (Chi² = 3.12, df = 3 (P = 0.37), I² = 3.8%). OS: Similar results were observed for overall survival with a HR of 1.91 (CI, 1.49-2.44, p < 0.00001) in favor of the auto-SCT group at relapse (Figure 1B). The test for heterogeneity showed marked heterogeneity between studies but, similar to PFS, did not reach statistical significance (Chi² = 6.28, df = 4 (P = 0.18), I² = 36.3%).
Discussion and Conclusion
The included studies showed some heterogeneity but the corresponding test did not reach a significance level. The main reasons for the differences between studies could be different ablative regimens and different patient characteristics. In some studies, the median age was lower in the Allo-SCT group, which favored it, a finding that was not observed in all comparative studies.
Allo-SCT appears to benefit only a small proportion of patients with multiple myeloma, whereas graft-versus-host disease and infectious complications affect outcome in a much larger proportion of patients, resulting in a worse prognosis for the entire allo-SCT group. This scenario is supported by our analysis showing a statistically significant advantage of a second auto-SCT over allo-SCT in terms of PFS and OS in patients who relapse after first-line auto-SCT.
Disclosures
Ludwig:Amgen, Sanofi: Research Funding; Sanofi, Takeda, Pfizer, Celgene-BMS, Oncopeptides, AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Atsuta:Otsuka Pharmaceutical Co., Ltd: Speakers Bureau; CHUGAI PHARMACEUTICAL CO., LTD.: Speakers Bureau; JCR Pharmaceuticals Co., Ltd.: Consultancy; Meiji Seika Pharma Co, Ltd.: Honoraria; Novartis Pharma KK: Speakers Bureau. Takamatsu:SRL: Consultancy; Janssen: Honoraria; Ono: Honoraria; Sanofi: Honoraria; Bristol-Myers Squibb: Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal